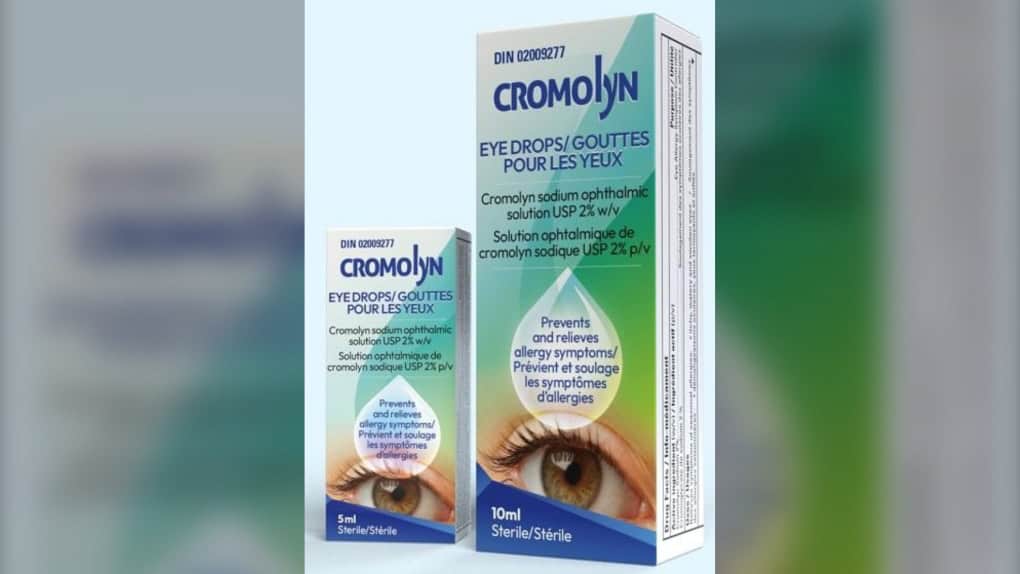
Cromolyn eye drops have been recalled in Canada due to potential microbial growth that may lead to eye infections.
Montreal-based Pendopharm, a division of Pharmascience Inc., is recalling 5 mL and 10 mL packs of its Cromolyn eye drops with the Drug Identification Number 02009277. The products have the following expiry dates: April 30, 2025; April 30, 2026; Nov. 30, 2026; Jan. 31, 2026; and Aug. 31, 2026.
The Canadian government advises that children, pregnant individuals, seniors, and those with weakened immune systems may be more susceptible to infections or complications from microbial contamination.
For healthy individuals, the risk of developing serious eye infections from potentially contaminated eye drops is considered “relatively low,” and such infections may resolve on their own.
The company recommends that consumers return the product to a pharmacy for proper disposal.
Those who want to contact the company can reach Pendopharm by calling 1-888-550-6060 or emailing medinfo@pendopharm.com.
As well, individuals can report any side-effects or complaints to Health Canada.